Background:
Post-transplant lymphoproliferative disease (PTLD), is one of the major complications after organ transplantation. The aim of this study is to study the incidence, various risk factors especially EBV status, histopathological types, management, and outcomes of PTLD following kidney transplantation in the pediatric groups.
Methods:
Following the PRISMA guideline, we performed a comprehensive literature search on PubMed, Cochrane Library, Embase, and clinicaltrials.gov from the past ten years on May 04, 2020. We used the MeSH terms of organ transplantation and lymphoproliferative disorders. The initial search revealed 1741 articles. We excluded all case reports, case series, pre-clinical trials, review articles, and meta-analysis. We found five retrospectives observational, one retrospective questionnaire survey, one prospective observational, and one prospective trial study. We extracted the data for baseline characteristics, the reason for transplantation, recipient & donor EBV status, immunosuppression used, type & stage of PTLD, organ system involved, duration between transplant and PTLD diagnosis, treatment, response to therapy, adverse effects of therapy and mortality.
Results:
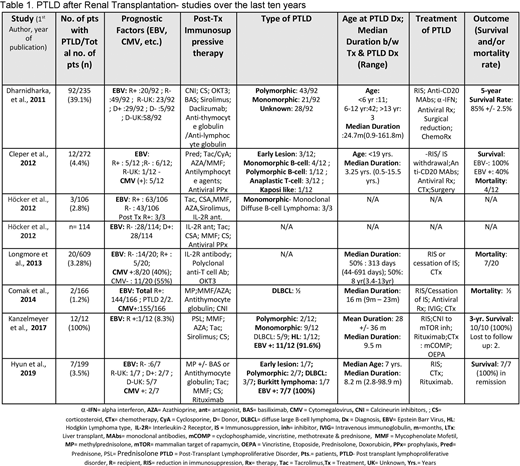
We included eight studies with a total (n) number of 1713 post-kidney transplant pediatric patients, out of which 148 (8.63%) patients who developed PTLD as a complication of transplantation were studied(table 1). Among the 148 patients diagnosed with PTLD, 96 (64.86%) were males, 49 (33.1%) female participants and 3 (2.2%) were unknown. 34/148 (22.97%) PTLD recipients were EBV (+), 86 (58.1%) EBV (-) and 28 (18.9%) unknown at the time of transplant. EBV status for donors was known in only 2 studies, showing 7/99 (7.1%) to be EBV (+) at the time of transplant. Höcker et al. have shown that antiviral prophylaxis with ganciclovir/valganciclovir in the first year post-renal transplant reduces the risk of EBV viremia. Post-transplant immunosuppressive drugs included tacrolimus, mycophenolate mofetil, azathioprine, sirolimus, cyclosporine, IL-2R antagonist, methylprednisolone, basiliximab, daclizumab, anti-thymocyte globulin/anti-lymphocyte globulin, OKT3. In some cohorts, rituximab and antiviral prophylaxis with ganciclovir or valganciclovir were also used in some patients. The median time from transplant to the diagnosis of PTLD from five studies with 125 patients was 16 months (0.9m-186m). Longmore et al. reported a bimodal distribution curve, with 50% presenting with early PTLD, i.e. after a median duration of 313 days and 50% presenting late after a median duration of 8 years. The histopathological types of PTLD were diagnosed via biopsy samples, showing predominance with polymorphic type 48 (32.4%), followed by monomorphic type 45 (30.4%), early lesion 4 (2.7%), Kaposi like PTLD 1 (0.67%) and Hodgkin lymphoma 1 (0.67%). The histological testing results from two of the studies also showed that 18/19 (94.7%) of diagnosed PTLD samples were EBV positive. PTLD was managed with reduction or cessation of the immunosuppressive drugs, anti-CD20 antibodies, chemotherapy for lymphoma, and in some cases mTOR inhibitors, intravenous immunoglobulins, and surgical resection. Data from 5 studies show the mortality rate of 12/51 (23.5%) among PTLD groups. The survival rate from 2 studies was 100% among 17 PTLD patients and 1 study showed a 5-year survival rate of 85% among 92 PTLD patients. Cleper et al. in their study concluded that the type of PTLD might have a significant effect on the outcome, as ¾ (75%) deaths in the PTLD group were attributed to anaplastic T-cell type.
Conclusions:
Our analysis shows the EBV infection is closely associated with a higher risk of PTLD development. Recipients' EBV seronegativity and positive EBV status of the donor have been shown to increase post-transplant EBV infection risk which is associated with a higher risk of PTLD development. Furthermore, our study shows that PTLD may occur in less than a month to more than 15 years of renal transplant. The polymorphic type was the most common and Hodgkin lymphoma-type, the least commonly reported PTLD type. The main therapeutic approach is the reduction or cessation of immunosuppression.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal